An Abbreviated Complimentary Analysis of U.S. Rules and Statutes
Point-of-Care Partners’ ePrescribing State Law Capsule is a quick reference table of U.S. laws and regulations governing ePrescribing. This complimentary resource, released quarterly, provides a consolidated view of ePrescribing laws in every state. Valuable for all ePrescribing stakeholders, the Law Capsule is an extract from our nationally-known, premium subscription product, the ePrescribing State Law Review.
Download the ePrescribing State Law Review Capsule flyer by CLICKING HERE.
ePrescribing State Law Capsule Features
The following data is included in the ePrescribing State Law Capsule quick reference table for every state.
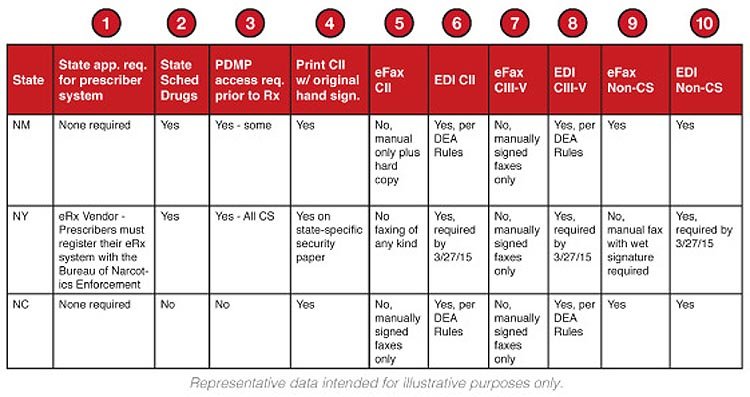
 State approval required for prescriber system
State approval required for prescriber system
Indicates whether the State Board of Pharmacy or other agency requires approval of the ePrescribing vendor or the transmission intermediary prior to operating in that state.
![]() State scheduled drugs
State scheduled drugs
If Yes, the state has specific drugs that are scheduled differently from the DEA schedule tables.
![]() PDMP access required prior to prescribing
PDMP access required prior to prescribing
If Yes, the prescriber is required to check the Prescription Drug Monitoring Database (PDMP) prior to writing a new prescription for controlled substances or other designated drugs of concern.
![]() Print Schedule II (CII) prescription with original hand signature
Print Schedule II (CII) prescription with original hand signature
If Yes, the state allows the prescriber’s signature to be electronically printed on Schedule II controlled substances prescriptions. Note: Although most states allow this, there are instances where the prescriber’s full handwritten prescription is required.
![]() eFax Schedule II (CII)
eFax Schedule II (CII)
Electronic faxing of controlled substances is no longer allowed, but this field indicates if any faxing is allowed for Schedule II drugs including manual fax with hard copy follow-up.
![]() EDI Schedule II (CII)
EDI Schedule II (CII)
If Yes, electronic prescribing of Schedule II controlled substances (EPCS) is allowed.
![]() eFax Schedule III-V (CIII-V)
eFax Schedule III-V (CIII-V)
If Yes, electronic faxes with electronic signatures are allowed for Schedule III-V controlled substances.
![]() EDI Schedule III-V (CIII-V)
EDI Schedule III-V (CIII-V)
If Yes, electronic prescribing of controlled substances (EPCS) of Schedule III-V (CIII-V) drugs is allowed.
![]() eFax Non-Controlled Substances
eFax Non-Controlled Substances
If Yes, electronic faxes with electronic signatures are allowed for non-controlled substances.
![]() EDI Non-Controlled Substances
EDI Non-Controlled Substances
If Yes, electronic prescriptions are allowed for non-controlled substances.
How can I be added to the distribution?
The ePrescribing State Law Capsule is distributed on a complimentary basis to industry stakeholders. To receive the most current edition and subsequent editions, subscribe now.
Do you need a more robust solution?
Our premium subscription product, The ePrescribing State Law Review, is a comprehensive data set that provides an in-depth analysis of State and Federal rules and statutes governing electronic prescriptions. It provides:
- All local output format requirements for faxed and printed prescriptions for each state
- Rules governing electronic transmission of prescriptions by e-fax or EDI
- Prescription authority rules and documentation requirements for mid-level prescribers
- State requirements for ePrescribing system approval—addresses, URLs and BOP executive contact information are provided
- Controlled Substance schedule variances by drug for states that categorize certain drugs differently from the DEA.
The Law Review is released quarterly, with additional interim updates to clients for breaking news alerts regarding important changes, and includes access to POCP’s regulatory experts to address specific client questions. To learn more about subscribing to the premium ePrescribing State Law Review, CLICK HERE.




