This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
HIT Perspectives: State Lawmakers Focus on ePA and Opioid Abuse
HIT Perspectives – June 2017
State Lawmakers Focus on ePA and Opioid Abuse

By Connie Sinclair, Senior Consultant
 Electronic prior authorization (ePA) and the nation’s opioid epidemic continue to capture the attention of state legislatures, many of which are considering or enacting laws related to these important topics. Here are some of the latest trends, as compiled by Point-of-Care Partners’ Regulatory Resource Center (RRC). The RRC helps clients stay up to date with frequent changes in state and national regulatory requirements through such service offerings as the ePrescribing State Law Review and the ePA State Navigator.
Electronic prior authorization (ePA) and the nation’s opioid epidemic continue to capture the attention of state legislatures, many of which are considering or enacting laws related to these important topics. Here are some of the latest trends, as compiled by Point-of-Care Partners’ Regulatory Resource Center (RRC). The RRC helps clients stay up to date with frequent changes in state and national regulatory requirements through such service offerings as the ePrescribing State Law Review and the ePA State Navigator.
Electronic prior authorization.
An increasing number of medications require preapproval — or prior authorization (PA) — from payers before they can be dispensed or administered. This traditionally has been a pain point for physicians and pharmacists, who have spent a lot of time in the frustrating exchange of paper, phone calls and faxes with payers only to find out their requests have been denied. This time lag obviously poses potential health and safety threats to patients.
Now that more care processes are becoming automated, the health care industry is beginning to move toward more widespread adoption of prospective ePA based on the standard from the National Council for Prescription Drug Programs (NCPDP). For additional information, see the article in this issue of HIT Perspectives.
States also are beginning to recognize the benefits of ePA based on the NCPDP standard. Eleven have enacted laws mandating payer support for prescriber-initiated NCPDP-formatted PA transactions. In a newly enacted law, Indiana is going a step further with requirements for ePA transactions initiated by pharmacists. Beginning January 1, 2018, health plans in the state must accept and respond to NCPDP-formatted ePA requests from a prescriber or dispensing pharmacist. Indiana’s statute does NOT mandate pharmacist use of this approach or say other methods are possible nor prohibited. Even so, this is another step toward promoting widespread adoption of ePA. States watch each other. Once the ice has been broken with respect to a particular regulatory approach, other states generally follow suit. In addition, an NCPDP workgroup is moving forward with development of a use case for ePA, based on the NCPDP standard, initiated by clinical pharmacists in the postacute care setting. Such activities should also hasten more widespread and complete adoption of prospective ePA. Stay tuned.
Opioid abuse.
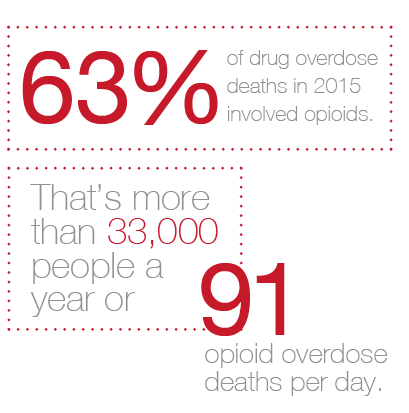
Measures aimed at stemming the tide of opioid-related deaths top the list of laws being passed or considered. This is not surprising. These powerful and highly addictive drugs are the leading cause of the drug overdose epidemic sweeping across communities nationwide. According to the Centers for Disease Control and Prevention, 63% of drug overdose deaths in 2015 involved opioids. That’s more than 33,000 people a year or 91 opioid overdose deaths per day.
Recent legislative efforts related to the prescribing of opioids fall into several categories. They include:
- Electronic prescribing of controlled substances. Several states — including Maine and Virginia — have joined New York to require electronic prescribing of all substances. Several other states have recently introduced similar legislation. However, specifics of the proposed mandates vary by state and will likely be amended as they are debated. Typically, there are long lead times for effective dates. Although various states will implement legislation related to opioid abuse over the next few years, there is no standard approach. Some laws will affect all controlled substances, some will deal with certain drug classes, while others will address specific drugs or drug schedules from the Drug Enforcement Administration.
- Prescription limitations. Several states have passed laws or regulations that place limits on prescription quantities for opioids. Not surprisingly, there are variations in terms of requirements. For example, prescription quantities are often set as a maximum number of days’ supply for the initial prescription. There can be different terms for subsequent orders, and these limits likely vary by diagnosis or circumstances and even by state. Increasingly, limits must be expressed in morphine milligram equivalents, which is something most electronic health record (EHR) vendors will need to build into their work flow. Ohio recently added some data requirements. That state’s board of pharmacy is requiring providers to place an ICD-10 or procedure code on all opioid prescriptions. This will help pharmacists better identify potential problems with a prescribed medication therapy and potential abuse. Such requirements have been suggested for years by policymakers, but Ohio is among the first states to mandate them.
- Consultation of state prescription drug monitoring databases. All states except Missouri have a prescription drug monitoring program (PDMP). PDMPs are independent, state-run databases of controlled substance prescriptions. Many states require prescribers to view a patient’s PDMP profile when prescribing. A few states are also specifically defining PDMP integration with EHRs to legally clear the way for data sharing among these implementations. Many leading EHR vendors are implementing PDMP access within their prescribing work flow.
Need to keep up with regulatory developments? Our RRC team members can help you quickly uncover answers to your most pressing regulatory questions. All clients in our premium portfolios receive complimentary access to our regulatory team members who are fully committed to providing the utmost quality in tracking and defining regulatory issues. Drop me an email (connie.sinclair@pocp.com) so we can show you how our expertise and data can meet your needs.



